RESEARCH
The research of our lab aims at understanding how stress exposure can lastingly affect brain function. Using animal models that allow for controlled study of the mechanistic underpinnings of stress-related psychopathology, we aim to elucidate how stress influences brain function at the molecular (investigating the epigenetic mechanisms and local gene expression) and neural circuit level (by means of neuronal activation markers, rodent MRI, viral tracing and optogenetics), while warranting translational value. Particular focus is put on the inter-individual differences in the neural correlates of stress responsivity and subsequent coping, by behavioral identification of stress resilient vs. susceptible subjects. The neural mechanisms underlying natural stress resilience may contain unique information for new treatment options for those suffering from stress-related mental illness.
Our research focuses along 3 main research lines :
1) Stress-induced imbalance in neural network function
Recent advances in the field of neuroscience made clear that proper stress recovery requires a complex interplay of functional brain networks, which' dysfunction or imbalance may ultimately result in stress-related disorders such as major depression or post-traumatic stress disorder (PTSD). In the healthy brain, neural resources are rapidly reallocated in response to acute stress/trauma exposure to prioritize emotional processing by the so-called salience network over the cognitive control network. During subsequent stress recovery, this reallocation is reversed (Hermans et al., 2014). Using rodent functional neuroimaging, analyzes of neuronal activity markers, as well as transgenic animal models, we are currently testing the hypothesis that PTSD may result from a chronic imbalance in neural network function (either present at baseline, occurring in response to trauma, or during recovery) in which salience processing continuously overrules cognitive function. Moreover, we are looking into the activity and connectivity of the renowned default mode network, which is also suggested to be upregulated in response to acute stress and dysregulated in stress-related mental disease (Henckens et al., 2015).
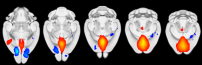
Own data demonstrating the existence of a default mode network in the mouse brain using resting-state fMRI.
Additional reading:
- Dirven BCJ, Negwer M, Botan A*, Maas R*, van Melis L*, Merjenburgh S*, van Rijn R*, Grandjean J^, Homberg JR^, Kozicz T^, Henckens MJAG (2024). The Neural Blueprint of Stress Susceptibility: Brain-wide neuronal activity associated with the consequences of stress. BioRxiv
- Henckens MJ, van der Marel K, van der Toorn A, Pillai AG, Fernández G, Dijkhuizen RM, Joëls M (2015). Stress-induced alterations in large-scale functional networks of the rodent brain. NeuroImage 105:312–22.
- Hermans EJ, Henckens MJ, Joëls M, Fernández G (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci 37(6): 304-14.
2) Epigenetic signature of trauma susceptibility
The mechanistic underpinnings of altered neuronal activity (as identified by research line 1) are further studied by focusing on the epigenetic mechanisms underlying gene expression differences. By making use of the recently developed transgenic FosTRAP and ArcTRAP mouse lines (Guenthner et al., 2013) - in which we can fluorescently label all Fos- or Arc-expressing neurons (ie, those active) - we can isolate neurons that are active at any given time-point (eg, before, during or after trauma). The activated neuronal populations can then be characterized by isolating the fluorescently labeled cells using fluorescence-activated cell sorting (FACS) allowing for the analysis of their molecular footprint.
Additional reading:
- Dirven BCJ, Homberg JR, Kozicz T, Henckens MJAG (2017). Epigenetic programming of the neuroendocrine stress response by adult life stress. J Mol Endocrinol 59(1): R11-31.
3) Memory engram for trauma
Intrusive memories of the trauma, such as flashbacks or recurrent nightmares, are among the most devastating symptoms of PTSD. Over-generalization of the traumatic memory has been suggested to underline these intrusive memories triggered by indiscriminate factors. Memory generalization has been related to deficits in hippocampus-mediated pattern separation, the process by which memories are stored as unique representations resistant to confusion. However, other research challenges this view and proposes that the abnormal memory trace in PTSD is caused by altered systems' memory processing instead. Aberrant amygdala activation in response to the trauma has been suggested to lead to altered configurations of neural activity and thereby qualitatively impact the formation of emotional memory representations. Using the FosTRAP and ArcTRAP mice in combination with brain clearing techniques (iDISCO+) and immunohistochemistry we are investigating these theories. Moreover, we are looking into the contribution of specific stress hormones (ie, corticosterone and norepinephrine) to the process of memory generalization.
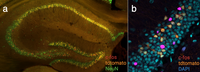
Own data showing fluorescent labeling of active neurons in the ArcTRAP mice. (a) Mouse brain slice in which neurons active during trauma encoding are labeled in red and those active during trauma recall in orange, counterstained with NeuN (anti c-FOS staining). (b) Zoomed in picture on the mouse hippocampus stained for c-FOS (magenta), tdtomato (orange) and counterstained with DAPI (blue). (c) Entire mouse brain hemisphere made transparent using iDISCO+, white dots representing active neurons tagged during trauma encoding (c-FOS staining).
Recent publications:
- Bahtiyar S, Karaca KG, Henckens MJAG, Roozendaal B. Exploring stress hormone effects on memory specificity and strength in mice using the dual-event inhibitory avoidance task. Learn Mem. 2025 Jan 17;32(1):a053956. doi: 10.1101/lm.053956.124. PMID: 39824646.
- Dirven BCJ, van Melis L, Daneva T, Dillen L, Homberg JR, Kozicz T, Henckens MJAG (2024). Hippocampal trauma memory processing conveying susceptibility to traumatic stress. Neurosci 540:87–102
- Grandjean J, … Henckens MJAG, … Hess A (2023). A consensus protocol for functional connectivity analysis in the rat brain. Nat Neurosci 26(6):1127–8
- Aberrant ventral dentate gyrus structure and function in trauma susceptible mice. Transl Psychiatry 12(1):502
- Longitudinal assessment of amygdala activity in mice susceptible to trauma. Psychoneuroendocrinology 145:105912
- Bosch K, Sbrini G, Burattini I, Nieuwenhuis D, Calabrese F, Schubert D, Henckens MJAG, Homberg JR (2022). Repeated testing modulates chronic unpredictable mild stress effects in male rats. Behav Brain Res 432:113960
- Mogavero F, Zwieten K, Buitelaar JK, Glennon JC, Henckens MJAG (2022). Deviant circadian rhythmicity, corticosterone variability and trait testosterone levels in aggressive mice. Eur J Neurosci 55(6):1492–1503
- Houtekamer MC, Henckens MJAG, van den Berg KP, Homberg J, Kroes MCW (2022). A Reminder Before Extinction Failed to Prevent the Return of Conditioned Threat Responses Irrespective of Threat Memory Intensity in Rats. Behav Neurosci 135(5):610–21
- Genzel L, .... Henckens MJAG, .... Homberg JR (2020). How the COVID-19 pandemic highlights the necessity of animal research. Current Biology 30(18):R1014-R1018.
- Bahtiyar S, Gulmez-Karaca K, Henckens MJAG, Roozendaal B (2020). Norepinephrine and glucocorticoid effects on the brain mechanisms underlying memory accuracy and generalization. Molecular and Cellular Neuroscience 108:103537.
- Preston G, Emmerzaal T, Kirdar F, Schrader L, Henckens MJAG, Morava E, Kozicz T (2020). Cerebellar mitochondrial dysfunction and concomitant multi-system fatty acid oxidation defects are sufficient to discriminate PTSD-like and resilient male mice. Brain, Behavior & Immunity - Health 6:100104.
- Song Q, Bolsius YG, Ronzoni G, Henckens MJAG, Roozendaal B (2020). Noradrenergic enhancement of object recognition and object location memory in mice. Stress [Epub ahead of print]
- Good vibrations: An observational study of real-life stress induced by a stage performance. Psychoneuroendocrinology 114:104593.
- Schipper P, Hiemstra M, Bosch K. Nieuwenhuis D, Adinolfi A, Glotzbach S, Borghans B, Lopresto D, Fernández G, Klumpers F, Hermans EJ, Roelofs K, Homberg JR, Henckens MJAG * (2019). The association between serotonin transporter availability and the neural correlates of fear bradycardia. Proceedings of the National Academy of Sciences of the United States of America 116(51):25941-25947. *: equal contributions
- Schipper P, Brivio P, de Leest D, Madder L, Asrar B, Rebuglio F, Verheij MMM, Kozicz T, Riva MA, Calabrese F, Henckens MJAG, Homberg JR (2019). Impaired fear extinction recall in serotonin transporter knockout rats is transiently alleviated during adolescence. Brain Sciences 9(5).
- Henckens MJAG *, Kroes MC*, Homberg JR (2019). How serotonin transporter gene variance affects defensive behaviors along the threat imminence continuum. Current Opinion in Behavioral Sciences 26: 25-31. *: equal contributions
- Schipper P, Henckens MJAG, Lopresto D, Kozic T, Homberg JR (2018). Acute inescapable stress relieves fear extinction recall deficits caused by serotonin transporter abolishment. Behavioral Brain Research 346:16–20.
- Schipper P, H enckens MJAG, Borghans B, Hiemstra M, Kozicz T, Homberg JR (2017). Prior fear conditioning does not impede enhanced active prevention in serotonin transporter knockout rats. Behavioral Brain Research 326:77–86.
- D irven BCJ, Homberg JR, Kozicz T, Henckens MJAG (2017). Epigenetic programming of the neuroendocrine stress response by adult life stress. J Mol Endocrinol 59(1): R11-31.
- van Bodegom M, Homberg JR, Henckens MJAG (2017). Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci 11:87.
- Henckens MJ, Printz Y, Shamgar U, Dine J, Lebow M, Drori Y, Kuehne C, Kolarz A, Eder M, Deussing JM. Justice NJ, Yizhar O, Chen A (2017). CRF receptor type 2 neurons in the posterior bed nucleus of the stria terminalis critically contribute to stress recovery. Mol Psychiatry 22(12): 1691-1700.
- Henckens MJ, Deussing JM, Chen A (2016). Region-specific roles of the corticotropin-releasing factor-urocortin system in stress. Nat Rev Neurosci 17(10):636–51.
- Henckens MJ, Klumpers F, Everaerd D, Kooijman SC, van Wingen GA, Fernández G (2016). Interindividual differences in stress sensitivity: basal and stress-induced cortisol levels differentially predict neural vigilance processing under stress. Soc Cogn Affect Neurosci 11(4):663–73.
- Schipper P, Lopresto D, Reintjes RJ, Joosten J, Henckens MJ, Kozicz T, Homberg JR (2015). Improved stress control in serotonin transporter knockout rats: Involvement of the prefrontal cortex and dorsal raphe nucleus. ACS Chem Neurosci 6(7):1143–50.
- Hermans EJ, Henckens MJ, Joëls M, Fernández G (2015). Toward a mechanistic understanding of interindividual differences in cognitive changes after stress: reply to van den Bos. Trends Neurosci 38(7): 403-4.
- Henckens MJ, van der Marel K, van der Toorn A, Pillai AG, Fernández G, Dijkhuizen RM, Joëls M (2015). Stress-induced alterations in large-scale functional networks of the rodent brain. NeuroImage 105:312–22.
- Pillai AG, Henckens MJ, Fernández G, Joëls M (2014). Delayed effects of corticosterone on slow afterhyperpolarization potentials in mouse hippocampal versus prefrontal pyramidal neurons. PLoS One 9(6):e99208.
- Hermans EJ, Henckens MJ, Joëls M, Fernández G (2014). Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci 37(6): 304-14.
- Find a list of all publications here .